Rat Subcutaneous Implant Model
Duration of study – Up to 30 days
Lead time – 30 days
Standard results – Microbiology, Histology
Model Utility:
The rat subcutaneous implant model is our most frequently requested (and most frequently recommended) animal model for screening antimicrobial and anti-biofilm actives and coatings. The model is fast, relatively inexpensive and, in our experience, 75-80% predictive of more complicated animal models. Using this model, it is possible to screen a wide array of microbial species with high reproducibility. Furthermore, as with all infection models, results are fast and unambiguous – actives that work generate significant log reductions compared to controls, as demonstrated in some of the examples below.
How The MoDel works:
example data:
Device is implanted subcutaneously (hernia mesh shown below)
Infection is added at exit site or through preculture of biofilm onto the material prior to implantation
Commonly requested bacteria are: MRSA, Staph epi., E. coli and Pseudomonas
Treatment can begin any time before or after implantation
Implanted device is collected for microbial counts & surrounding tissue is evaluated histologically
Study duration can be hours to at least 6 months
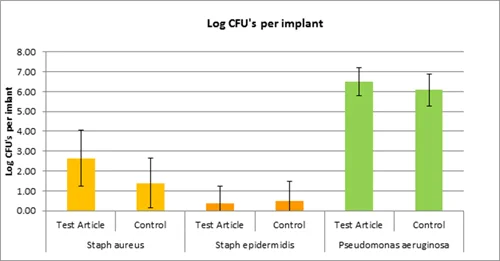
The following are real data generated during studies. Due to confidentiality, we cannot disclose any of the test articles being evaluated, but we have described them as part of different "classes" of actives that we are often asked to evaluate.
Example Experiment 1:
"Antimicrobial coated implant"
6538 = ATCC strain of wt Staph (ATCC 6538)
Example Experiment 2: "Anti-adhesive coated implant"
Example Experiment 3:
"Injectable antimicrobial"
pathology is always available upon request:
Gram stain of the infected implant shows presence of S. aureus biofilm on device surface.